Ap Biology Gibbs Free Energy Video Review Sheet
All AP Chemistry Resources
Suppose that a rxn has ∆H = -28 kJ and ∆S= -60 J/K. At what temperature will it
change from spontaneous to non-spontaneous?
Explanation:
Approximately 467 K. ∆G=∆H-T∆S and a rxn proceeds spontaneously when ∆G < 0
and is non-spontaneous when ∆Grand > 0. And so if we set ∆G=0 and solve the equation for T, we will see that the crossover from spontaneous to non-spontaneous occurs when T=467K.
If the reaction quotient (Q) is greater than the equilibrium abiding (K), what is true about the Gibbs free energy?
Possible Answers:
It is equal to nil.
More than information is needed to determine the gibbs free energy.
Information technology is greater than cipher.
Information technology is less than zero.
Right answer:
Information technology is greater than zero.
Explanation:
If Q is greater than Yard, the reaction has exceeded the equilibrium state. It volition continue nonspontaneously (since equilibrium has already been reached), and this must mean that the ΔG (gibbs costless energy) must be positive, or greater than cypher.
The entropy and enthalpy of a reaction are both negative. Is the reaction spontaneous?
Possible Answers:
The reaction volition be spontaneous if and just if the magnitude of the enthalpy is greater than the magnitude of the entropy times the temperature
The reaction will be spontaneous if and only if the magnitude of the enthalpy is greater than the magnitude of the entropy
The reaction will exist spontaneous if and only if the magnitude of the entropy is greater than the magnitude of the enthalpy times the temperature
The reaction is spontaneous
The reaction is not spontaneous
Correct answer:
The reaction will be spontaneous if and only if the magnitude of the enthalpy is greater than the magnitude of the entropy times the temperature
Caption:
A reaction is spontaneous if the Gibb's Free Energy of the reaction is negative.

If 

A system with __________ and__________ will never be spontaneous.
Possible Answers:
positive enthalpy . . . negative entropy
positive enthalpy . . . positive entropy
negative enthalpy . . . positive entropy
negative enthalpy . . . negative entropy
Right answer:
positive enthalpy . . . negative entropy
Caption:
The Gibb's costless energy equation is used to determine the spontaneity of a reaction and is written as follows: 





This means these are the weather condition that volition always result in a nonspontaneous reaction.
Consider the following reaction in a galvanic cell:
Which if the following statements about the reaction is false?
Possible Answers:
The equilibrium constant is greater than one
The reaction is spontaneous
Gibb's free energy is positive
The jail cell potential is positive
Correct answer:
Gibb'southward free energy is positive
Explanation:
A galvanic cell results in a positive cell potential from a spontaneous reaction. Spontaneous reactions always have a negative Gibb's free energy.
An equilibrium abiding greater than one would indicate that the equilibrium concentration of products is greater than the equilibrium concentration of reactants, consistent with a spontaneous reaction.
Consider the following reaction in a galvanic cell:
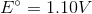
What is the Gibb'southward free energy for the reaction under standard conditions?

Correct respond:

Explanation:
You can discover the Gibb's free energy of a galvanic cell by using the following equation:




We are given the constant value and the prison cell potential. The moles of electrons transferred is equal to the alter in charge on the atoms in the reaction. In this reaction, copper is reduced from a charge of 
Using these values, we tin observe Gibb'due south costless energy for the jail cell.


Discover that the value is negative. Galvanic cells are spontaneous, and will have negative Gibb's free energies at standard weather.
If the reaction quotient (Q) is less than the equilibrium constant (K), what is true most the Gibbs free energy?
Possible Answers:
Information technology is less than zero
Information technology is equal to zero
Nosotros must know the reaction temperature to make up one's mind the answer
It is greater than zero
We must know the reaction enthalpy to decide the answer
Correct answer:
Information technology is less than zero
Explanation:
If Q is less than K, then the reaction has not yet reached the equilibrium land. It volition proceed spontaneously in the forward direction. Since it is proceeding spontaneously in the forrard direction, this must mean that the ΔG (Gibbs energy) must exist negative, or less than zero.
Enthalpy and temperature are necessary when mathematically evaluating Gibbs free energy, but the reaction quotient and equilibrium constant provide enough data to qualitatively determine that the reaction is proceeding spontaneously.
Of the following reactions, which of the following is only spontaneous at high enough temperatures?
Possible Answers:
∆H –, ∆S +
∆H +, ∆S –
∆H +, ∆S +
∆H –, ∆S –
None of the Above
Correct answer:
∆H +, ∆S +
Explanation:
Based on Gibbs Gratuitous Energy, ∆G = ∆H – T∆S, nosotros find that ∆H– volition contribute to spontaneity (∆G<0). Since we are looking at temperatures, in order for ∆S to make an nonspontaneous reaction spontaneous at but high temperatures, ∆H will exist positive, and ∆Southward will be positive.
If a reaction has a positive value for its enthalpy and a negative value for its entropy, which of the following is true?
Possible Answers:
The reaction is at equilibrium
The reaction is spontaneous
Not enough data to tell
The reaction is nonspontaneous
Correct answer: The reaction is nonspontaneous
Explanation:
Given the equation for gratuitous energy, (delta)G = (delta)H-T(delta)S, nosotros can determine that the reaction is nonspontaneous at all temperatures if H is positive and South is negative. This combination would always lead to a positive One thousand value, meaning that complimentary energy is required for the reaction to take place and information technology is therefore nonspontaneous.
What must be truthful of a spontaneous process?
Possible Answers:
ΔG → 0 and K → Q
ΔG → 0 and K ← Q
1000 ⇌ 0
ΔG ← 0 and Chiliad → Q
ΔG ← 0 and One thousand ← Q
Correct answer:
ΔG ← 0 and K → Q
Explanation:
Change in free energy must always be negative for a spontaneous process. Additionally, Q must exist less than G and so that the reaction will proceed in the forward reaction, toward equilibrium.
All AP Chemistry Resources
Report an consequence with this question
If you've institute an issue with this question, delight permit us know. With the help of the community we tin can continue to ameliorate our educational resource.
DMCA Complaint
If you believe that content available by means of the Website (as defined in our Terms of Service) infringes i or more of your copyrights, please notify us by providing a written observe ("Infringement Detect") containing the data described below to the designated agent listed below. If Varsity Tutors takes activity in response to an Infringement Notice, it will make a good organized religion endeavour to contact the party that made such content available past means of the near recent email accost, if any, provided past such political party to Varsity Tutors.
Your Infringement Notice may be forwarded to the party that made the content bachelor or to third parties such as ChillingEffects.org.
Please be brash that yous will exist liable for damages (including costs and attorneys' fees) if you materially misrepresent that a production or activeness is infringing your copyrights. Thus, if yous are non certain content located on or linked-to past the Website infringes your copyright, you should consider outset contacting an chaser.
Please follow these steps to file a notice:
You lot must include the following:
A physical or electronic signature of the copyright owner or a person authorized to act on their behalf; An identification of the copyright claimed to have been infringed; A clarification of the nature and exact location of the content that you claim to infringe your copyright, in \ sufficient detail to permit Varsity Tutors to find and positively place that content; for case nosotros crave a link to the specific question (non only the name of the question) that contains the content and a description of which specific portion of the question – an epitome, a link, the text, etc – your complaint refers to; Your name, address, telephone number and email address; and A statement past y'all: (a) that you believe in good faith that the use of the content that you merits to infringe your copyright is not authorized by constabulary, or by the copyright possessor or such owner's agent; (b) that all of the information contained in your Infringement Notice is accurate, and (c) nether penalty of perjury, that yous are either the copyright owner or a person authorized to human action on their behalf.
Send your complaint to our designated agent at:
Charles Cohn Varsity Tutors LLC
101 S. Hanley Rd, Suite 300
St. Louis, MO 63105
Or make full out the class below:
winsordidessair73.blogspot.com
Source: https://www.varsitytutors.com/ap_chemistry-help/gibbs-free-energy-and-spontaneity

0 Response to "Ap Biology Gibbs Free Energy Video Review Sheet"
Post a Comment